@alan
This article is where to start https://academic.oup.com/pcp/article/50/1/2/1851992
(https://academic.oup.com/pcp/article/50/1/2/1851992#skipNav)
" JOURNAL ARTICLE
Plant Nutrition—Roots of Life for Fundamental Biology and Better Crop Production
[Toru Fujiwara](javascript:;), [Toru Matoh](javascript:
Plant and Cell Physiology, Volume 50, Issue 1, January 2009, Pages 2–4, https://doi.org/10.1093/pcp/pcn195
Published:
15 January 2009
(https://academic.oup.com/pcp/search-results?f_TocHeadingTitle=Editorial)
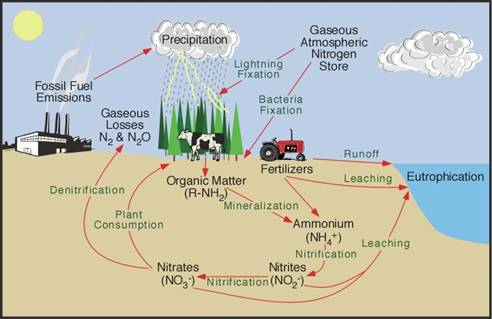
The world’s population reached 6.7 billion in 2008 and continues to grow. 2008 was also a year marked by high food prices, and indeed food crises have arisen in developing countries. Given the increase in population and decrease in available arable land, the public expects us, the plant science community, to provide technologies that maintain and increase food production. Plants grow in the soil, take up mineral nutrients and generate food for us. Therefore, the uptake of mineral nutrients by plants from the soil is a critical step, in terms of both food production and global element cycling.
The human body contains about 1.5 kg of nitrogen atoms. Every human eats nearly three times this quantity of nitrogen every year in the form of protein, equivalent to 73 g of protein a day. The current world population consumes some 28 million tonnes of protein-nitrogen every year. Eighty-five percent of the nitrogen in food proteins comes from agriculture, either directly in plant-derived foods or indirectly via animals fed with plant material. Synthetic fertilizers derived from the Haber–Bosch synthesis of ammonia provide 44–51% of all the nitrogen absorbed by crops. Therefore, roughly 40% of nitrogen in foods derives from synthetic ammonia (Smil [2002](javascript:;)). Nitrogen fertilizers were first introduced using the few natural deposits of nitrate salts such as Chilean nitrate, and as ammonium sulfate produced as a by-product from gasworks, but the Haber process was established on an industrial scale in 1913 and has since ensured unlimited supplies from atmospheric nitrogen (Jenkinson [2001](javascript:;)).
These figures indicate that nitrogen fertilizers are absolutely essential for human life on earth. Borlaug summed up the role that N fertilizers played in the Green Revolution by using a memorable kinetic analogy: ‘If the high-yielding dwarf wheat and rice varieties are the catalysts that have ignited the Revolution, then chemical fertilizer is the fuel that has powered its forward thrust’ (Borlaug 1970 cited by Smil [2002](javascript:;)). Even though the requirement will increase further as the world’s population grows, research into nitrogen metabolism in the soil has barely begun.
It is known that rice grain yield is a function of nitrogen uptake by rice plants, and a larger yield is attained only under higher nitrogen uptake (Haefele et al. [2008](javascript:;)). On the other hand, it is also appreciated that the absolute efficiency of nitrogen uptake varies according to rice variety, and is thus controlled genetically. The molecular mechanism underlying this has not been fully revealed, even though all the enzymes involved in nitrate reduction and ammonium assimilation have been identified, along with their corresponding genes and several other regulatory genes. A more or less similar situation exists for other fertilizers, including P and K. Low-input, high yield production is desirable given the limited resources of fertilizers and energy consumption in production, transportation and application of fertilizers.
Plant nutrition is a complex process that has developed over the course of plant evolution. Plants support our life by extending their leaves into the air and their roots in the soil. Roots take up nutrients from the soil and transport them to the leaves to support photosynthesis. Most soils are poor in nutrients, and plants have evolved accordingly, regulating their transport systems depending on the nutritional conditions. In many cases, nutrient deprivation induces high affinity uptake systems. The development of roots and leaves is also influenced by nutritional conditions. In particular, it is well known that the root/shoot ratio and lateral root development are regulated by nutrition. For such regulatory systems to function, nutrient conditions need to be sensed, signals need to be transduced, gene expression needs to be transcriptionally and post-transcriptionally regulated, transporters must be properly trafficked through endomembrane systems, and cell cycles and cell elongation need to be coordinated. Metabolism is also under the influence of nutritional conditions. Such a wide range of responses may be a reflection of the very sophisticated systems that have evolved in plants over time. In other words, a proper understanding of plant nutrition requires an understanding of all of these processes.
In the last few decades, the field of plant nutrition has advanced rapidly, incorporating a wide range of plant sciences. At the same time, plant nutrition research has enormous potential to contribute to other plant and biological sciences. For example, the first boron transporters in living systems were identified in plants, and human and yeast boron transporters were identified subsequently (Takano et al., [2002](javascript:;)). This transporter also contributed to the understanding of endomembrane trafficking systems (Takano et al. [2005](javascript:;)). The recent discovery of silicon transporters and their subcellular localization attracted much attention (Ma et al. [2006](javascript:;), Ma et al. [2007](javascript:;)). Another recent breakthrough in plant nutrition was the successful generation of plants that tolerate nutritional stresses (Takahashi et al. [2001](javascript:;), Yanagisawa et al. 2002, Miwa et al. [2006](javascript:;), Ishimaru et al. [2007](javascript:;), Miwa et al. [2007](javascript:;)). Transgenic rice lines engineered to be tolerant to iron deficiency were successfully tested in the field (Kobayashi et al. [2008](javascript:;), Suzuki et al. [2008](javascript:;)). Generation of nutrient stress-tolerant crops will contribute to increase yields; but, to achieve this goal, a wide range of nutrient studies need to be conducted and coordinated among the research community.
The demand for increased food production has a long history, and plant nutrition has played significant roles in responding to the challenge. Indeed, from soil and plant diagnosis to suggestions for appropriate fertilizer applications, current levels of food production would never have been possible without knowledge of plant nutrition. In developed countries where fertilization is optimized, however, traditional plant nutrition approaches can no longer boost food production any further. By incorporating new knowledge and technologies, the field of plant nutrition has reached a new level where crop production can potentially be improved without significant application of fertilizers. Such research will give us insights into not only plant physiology, but also the ways in which we can manipulate plants for better production.
In this special issue, we asked innovative plant nutritionists to report on their exciting work. All the manuscripts were peer reviewed. First, Mitani et al. describe silicon transporters in maize (pp. 5–12). This is an extension of the group’s discovery of silicon transporters in rice. The silicon transporter is essential for normal growth of rice, and its interesting polar localization revealed the importance of membrane trafficking in plant nutrient transport. In the present study, silicon transporters in maize are described, extending the understanding of silicon transport mechanisms in crop plants. Next, Yuan et al. describe a pollen-specific ammonium transporter in Arabidopsis (pp. 13–25). The group has characterized ammonium transporters and the manuscript describes the unique cell-specific expression of an uncharacterized member of the ammonium transporter family. Koshiba et al. describe the involvement of oxidative stresses in cell death following boron deprivation (pp. 26–36). The group has made a significant contribution to boron physiology, especially regarding the roles of boron in cell walls. The present study may lead to a novel strategy to generate plants tolerant to low B conditions. Sawada et al. describe a metabolomic analysis of a number of plant species and characterize their properties (pp. 37–47). It is obvious that metabolism is an important area in plant science, but, due to the technical challenges of analysis, only a limited number of metabolites have been analyzed. Their report is a good example of how a novel research technology can contribute to our understanding of plant metabolism. Tsukamoto et al. describe real-time tracer analysis to visualize Fe movement in barley (pp. 48–57). This technology allows us to understand the movement of nutrients throughout plant development, which has not been possible until recently. Finally, Kato et al. describe the successful development of plants highly tolerant to low B conditions, through manipulation of boron transporters (pp. 58–66). Together, these articles cover a wide range of plant nutritional studies representing the current status of the field.
It is our view that with the recent demand for high quality laboratory research exploiting novel technologies, the importance of field science tends to be ignored. It is certainly important to understand more deeply how plants function, but we believe that there is no future for ‘in vitro’ plant biology on its own. Without accompanying knowledge generated under field conditions, we could not transfer our new understanding to real agriculture. We strongly believe that this final application is essential if we hope to answer the public’s expectations. I hope this special issue will be perceived as an invitation for a wide range of plant scientists to focus their attention on plant nutrition, and that it will stimulate a wider exchange of ideas and expertise. We also hope that it will encourage the submission of more excellent studies to Plant and Cell Physiology in the field of nutrition in the future.
"
You already know all that but others may not but the cec cycle is how it all works. The good references like acres usa or more basic ones like soul of the soil by grace gershuny explain the cycle better.
https://www.sciencedirect.com/topics/engineering/cation-exchange-capacity
" 6.6.2.4 Cation exchange capacity and nutrient status
CEC measures the soil’s ability to hold or store positively charged ions. It is a very important soil feature that influences structural stability, nutrient availability, pH, fertilizer, and other ameliorants reaction of the soil (Hazelton and Murphy, 2007). CEC of a soil is dependent on the amount and type of soil colloids, clay minerals, and soil organic matter. It also reflects the total negative charge exchange sites on soil colloids (Follett, 2001) and increases correspondingly with pH, % clay, and % organic matter content. Improvement of soil nutrient by NPs is partly linked with their ability to increase CEC (Selva-Preetha and Balakrishnan, 2017). Improvement of CEC of soil at varying pH by nanozeolite has been confirmed (Qin et al., 2016; Chi et al., 2017). Fitriatin et al. (2017) linked the CEC increase in soils treated with volcanic ash NPs to increased negative charges contributed by silicate ions from NPs. High CEC enhances nutrient release in soil and also influences clay dispersibility and the concentration of soil colloids (Itami and Kyuma, 1995). Apart from the direct influence of NPs on soil CEC, the direct toxic effects on soil microbial enzymes may affect the nutrient status of soils (Eivazi et al., 2018). The authors reported that Ag NPs reduced the activities of acid phosphatase, β-glucosaminidase, β-glucosidase, and arylsulfatase regardless of size of the particles."
This is how it ties in to the plants https://www.ctahr.hawaii.edu/mauisoil/c_relationship.aspx#:~:text=Nutrient%20ions%20move%20into%20the,the%20root%2C%20called%20cortex%20cells.
" 
Home Soil Basics Soils of Maui Nutrient Management References

Soil-Nutrient Relationships
Cation exchange
The ‘soil cations’ essential for plant growth include ammonium, calcium, magnesium, and potassium. There are three additional ‘soil cations,’ which are not essential plant elements but affect soil pH. The additional ‘soil cations’ include sodium, aluminum and hydrogen.
Soil cations that are essential to plant growth
- Ammonium
- Calcium
- Magnesium
- Potassium
Soil cations that affect soil pH
The major distinguishing characteristic of cations is their positive charge. Just like a magnet, a positive charge is strongly attracted to a negative charge. When soil particles have a negative charge, the particles attract and retain cations. These soils are said the have a cation exchange capacity. Although most soils are negatively charged and attract cations, some Hawaii soils are exceptions as we will see.
The ‘soil cations’ are further divided into two categories. Ammonium, calcium, magnesium, potassium, and sodium are known as the ‘base cations,’ while aluminum and hydrogen are known ‘acid cations.’
Base Cations
Acid Cations
The words ‘base’ and ‘acid’ refer to the particular cation’s influence on soil pH. As you might suspect, a soil with a lot of acid cations held by soil particles will have a low pH. In contrast, a highly alkaline soil predominately consists of base cations.
Cations in the soil compete with one another for a spot on the cation exchange capacity. However, some cations are attracted and held more strongly than other cations. In decreasing holding strength, the order with which cations are held by the soil particles follows: aluminum, hydrogen, calcium, potassium and nitrate, and sodium.
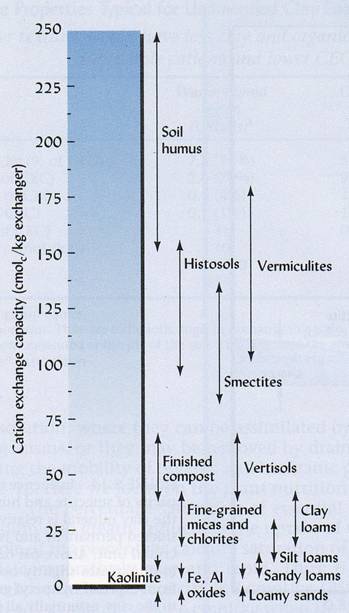
Figure 2. CEC values of various soil type, media, and minerals. Soils which have high amounts of organic matter and moderately weathered clays tend to have high CECs. As soils become highly weathered, the CEC of the soil decreases. Sandy soils, too, generally have lower CEC values. This is due to the lesser surface of sandy particles in comparison with clay minerals, which decreases the ability of sand particles to hold and retain nutrients.
Source: Brady and Weil. 2002. Elements of the Nature and Properties of Soil. Prentice Hall, New Jersey.
Anion exchange
In the tropics, many highly weathered soils can have an anion exchange capacity. This means that the soil will attract and retain anions, rather than cations. In contrast to cations, anions are negatively charged. The anions held and retained by soil particles include phosphate, sulfate, nitrate and chlorine (in order of decreasing strength). In comparison to soils with cation exchange capacity, soils with an anion capacity have net positive charge. Soils that have an anion exchange capacity typically contain weathered kaolin minerals, iron and aluminum oxides, and amorphous materials. Anion exchange capacity is dependent upon the pH of the soil and increases as the pH of the soil decreases.
Base Saturation
Base saturation is a measurement that indicates the relative amounts of base cations in the soil. By definition, it is the percentage of calcium, magnesium, potassium and sodium cations that make up the total cation exchange capacity. For example, a base saturation of 25 % means that 25 % of the cation exchange capacity is occupied by the base cations. If the soil does not exhibit an anion exchange capacity, the remainder 75 % of the CEC will be occupied by acid cations, such as hydrogen and aluminum. Generally, the base saturation is relatively high in moderately weathered soils that formed from basic igneous rocks, such as the basalts of Hawaii. The pH of soil increases as base saturation increases.
In contrast, highly weathered and/or acidic soils tend to have low base saturation.
Movement of nutrient from soil to root
There are three basic methods in which nutrients make contact with the root surface for plant uptake. They are root interception, mass flow, and diffusion.
- Root interception: Root interception occurs when a nutrient comes into physical contact with the root surface. As a general rule, the occurrence of root interception increases as the root surface area and mass increases, thus enabling the plant to explore a greater amount of soil. Root interception may be enhanced by mycorrhizal fungi, which colonize roots and increases root exploration into the soil. Root interception is responsible for an appreciable amount of calcium uptake, and some amounts of magnesium, zinc and manganese.
- Mass flow: Mass flow occurs when nutrients are transported to the surface of roots by the movement of water in the soil (i.e. percolation, transpiration, or evaporation). The rate of water flow governs the amount of nutrients that are transported to the root surface. Therefore, mass flow decreases are soil water decreases. Most of the nitrogen, calcium, magnesium, sulfur, copper, boron, manganese and molybdenum move to the root by mass flow.
- Diffusion: Diffusion is the movement of a particular nutrient along a concentration gradient. When there is a difference in concentration of a particular nutrient within the soil solution, the nutrient will move from an area of higher concentration to an area of lower concentration. You may have observed the phenomenon of diffusion when adding sugar to water. As the sugar dissolves, it moves through parts of the water with lower sugar concentration until it is evenly distributed, or uniformly concentrated. Diffusion delivers appreciable amounts of phosphorus, potassium, zinc, and iron to the root surface. Diffusion is a relatively slow process compared to the mass flow of nutrients with water movement toward the root.
Nutrient Uptake into the root and plant cells
Before both water and nutrients are incorporated into plants, both must first be absorbed by plant roots.
UPTAKE OF WATER AND NUTRIENTS BY ROOTS
- Root hairs, along with the rest of the root surface, are the major sites of water and nutrient uptake.
- Water moves into the root through osmosis and capillary action.
- Soil water contains dissolved particles, such as plant nutrients. These dissolved particles within soil water are referred to as solute. Osmosis is the movement of soil water from areas of low solute concentration to areas of high solute concentration. Osmosis is essentially the diffusion of soil water.
- Capillary action results from water’s adhesive (attraction to solid surfaces) and cohesion (attraction to other water molecules). Capillary action enables water to move upwards, against the force of gravity, into the plant water from the surrounding soil.
- Nutrient ions move into the plant root by diffusion and cation exchange.
- Diffusion is the movement of ions along a high to low concentration gradient.
- Cation ion exchange occurs when nutrient cations are attracted to charged surface of cells within the root, called cortex cells. When cation exchange occurs, the plant root releases a hydrogen ion. Thus, cation exchange in the root causes the pH of the immediately surrounding soil to decrease.
- Once water and nutrient ions enter the plant root, they move though spaces that exist within the root tissue between neighboring cells.
- Water and nutrients are then transported into the xylem, which conducts water and nutrients to all parts of the plant.
Once water and nutrients enter the xylem, both can be transported to other parts in the plant where the water and nutrients are needed. The basic outline of how nutrient ions are absorbed by plant cells follows.
ABSORPTION OF NUTRIENTS INTO PLANT CELLS
- Plant cells contain barriers (plasma membrane and tonoplast) that selectively regulate the movement of water and nutrients into and out of the cell. These cell barriers are:
- permeable to oxygen, carbon dioxide, as well as certain compounds.
- semi-permeable to water.
- selectively permeable to inorganic ions and organic compounds, such as amino acids and sugars.
- Nutrient ions may move across these barriers actively or passively
- Passive transport is the diffusion of an ion along a concentration gradient. When the interior of the cell has a lower concentration of a specific nutrient than the outside of the cell, the nutrient can diffuse into the cell. This type of transport requires no energy.
- Active transport is the movement of a nutrient ion into the cell that occurs against a concentration gradient. Unlike passive transport, this type of movement requires energy.
Nutrient Mobility
WITHIN PLANT
An important characteristic of some nutrients is the ability to move within the plant tissue. In general, when certain nutrients are deficient in the plant tissue, that nutrient is able translocate from older leaves to younger leaves where that nutrient is needed for growth. Nutrients with this ability are said to be mobile nutrients, and include nitrogen, phosphorus, potassium, magnesium, and molybdenum. In contrast, immobile nutrients do not have the ability to translocate from old to new growth. Immobile nutrients include calcium, sulfur, boron, copper, iron, manganese, and zinc.
Nutrient mobility, or immobility, provides us with special clues when diagnosing deficiency symptoms. If the deficiency symptom appears first in the old growth, we know that the deficient nutrient is mobile. On the other hand, if the symptom appears in new growth, the deficient nutrient is immobile.
WITHIN THE SOIL
Mobility of a nutrient within the soil is closely related to the chemical properties of the soil, such as CEC and AEC, as well as the soil conditions, such as moisture. When there is sufficient moisture in the soil for leaching to occur, the percolating water can carry dissolved nutrients which will be subsequently lost from the soil profile. The nutrients which are easily leached are usually those nutrients that are less strongly held by soil particles. For instance, in a soil with a high CEC and low AEC, nitrate (an anion) will leach much more readily than calcium (a cation). Additionally, in such a soil, potassium (a monovalent cation) will leach more readily than calcium (divalent cation) since calcium is more strongly held to the soil particles than potassium.
Silica from minerals also dissolves and leaches from the soil profile during the processes of weathering. It is this dissolution and leaching that transforms primary minerals to the more weathered, secondary minerals that make up the finely-textured soils of Maui.
<< Previous Next >>
The University of Hawai‘i is an equal opportunity/affirmative action institution.
copyright ©2007-2022 University of Hawai‘i - College of Tropical Agriculture and Human Resources"
@alan
The part i’m referring to is actually the Movement of nutrient from soil to root. Since my soil is high in ph by adding nutrients in the form of compost which is negatively charged more cations can be taken up by the roots. Your soil is acidic so as you mentioned this is not particularly valuable to you. In Kansas or any place with a higher ph soil this is very valuable information. This is all things you know but maybe not heard expressed in this way.This section explains in more detail what is meant for your area but please ignore this was written for hawaii. Before someone asks how i know your ph it is based on your posts blueberries are a major crop for you. Blueberries only grow in very acidic annion rich soil not in my cation rich alkaline soil.
" 
Home Soil Basics Soils of Maui Nutrient Management References
 Home > Nutrient Management > Soil Acidity and Liming
Home > Nutrient Management > Soil Acidity and Liming
Soil acidity and liming
Soil pH is a useful indicator of the relative acidity or alkalinity of a soil. The pH scale ranges from 0 to 14, and the soil is assigned a value from the pH scale to describe the acidity or alkalinity. Since pH 7 falls midway along the scale, pH values that are equal to 7 are said to be neutral. However, pH values that fall below 7 are acidic, while pH values above 7 are alkaline.
By definition, the pH of a soil is the measurement of the concentration of hydrogen ions in soil water. Recall that the hydrogen ion is an acid cation. The greater the concentration of hydrogen ions in the soil water solution, the lower the pH. In return, the lower the pH value, the greater the acidity of the soil will be. The concentration of hydrogen ions in the soil solution is directly proportionate to and in equilibrium with the hydrogen ions retained on the soil’s cation exchange complex. Thus, the hydrogen ions retained by clay particles replenish, or buffer, the hydrogen ions in soil water.
Table 1. pH of some common items.
Item pH Item pH
Most acid soils 4.0 - 6.0 Lemon juice 2.2 - 2.4
Orange juice 3.4 - 4.0 Vinegar 4.0 - 4.5
Acid rain 3.0 - 5.0 Clean rain water 5.5 - 5.7
Fresh milk 6.3 - 6.6 Blood plasma 7.2 - 7.4
Mild soap solution 8.5 - 10.0
Source: Hue, N.V. and Ikawa, I. Acid Soils in Hawaii: Problems and Management. CTAHR.
http://www.ctahr.hawaii.edu/huen/hue_soilacidity.htm
Figure 3. pH values of common substances.
Soil pH is an important soil property, because it affects the chemical, biological, and physical processes of the soil. Thus, pH is often considered the “master variable” of soil. Its importance in nutrient management cannot be understated. To understand the significance of pH, its effects are listed below:
Effects of soil pH:
NUTRIENT AVAILABILITY
- Controls the availability of the essential nutrients
- Availability of nitrogen, phosphorus, sulfur, calcium, magnesium, sodium, and molybdenum is limited under acidic conditions
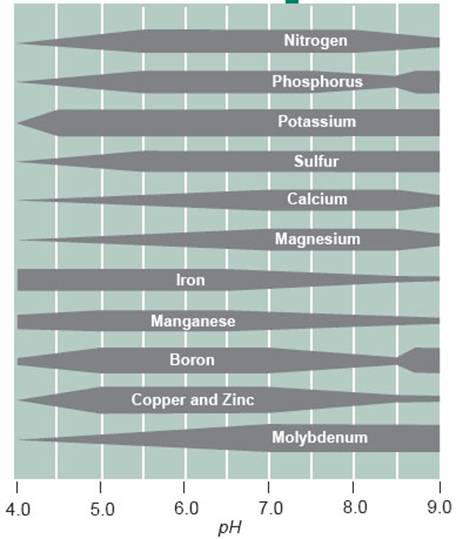
Figure 4. The effect of soil pH on the availability of essential plant elements. Greater nutrient availability is indicated by thickened lines, whereas narrow lines indicate a decrease in availability.
BIOLOGICAL ACTIVITY AND PROCESSES
- Determines the abundance of soil microorganism
- Determines which plant species will grow
- Low soil pH slows the biological transformation of ammonium to nitrate
PHYSICALLY
- Indirectly, high pH can disrupt soil structure, or aggregation.
Origins of acidity
There are a multiple origins of soil acidity. The following is a list of causes which are common in Hawaii:
-
Release of hydrogen atoms under natural chemical processes in the soil
- Atmospheric carbon dioxide reacts with water to form carbonic acid
- Organic molecules react with water and cause acid dissociation
- Oxidation of ammonium nitrogen, sulfur, and iron
-
Accumulation of organic matter and subsequent release of fulvic and humic acid, products of decomposition
-
Reaction of aluminum cations with water (a process known as hydrolysis)
-
Natural Deposition
- Lightning deposits acidic HNO3
- Volcanic activity deposits acidic H2SO4
-
Human Factors
- Oxidation of applied synthetic ammonium based fertilizers
- Oxidation of nitrogen compounds in applied animal manures and/or sewage sludge
- Deposition of acid rain (HNO3 and H2SO4) caused by industrial pollution
Pools of Soil Acidity
There are three general pools, or sources, of acidity: active, exchangeable or residual.
-
Active acidity is the quantity of hydrogen ions that are present in the soil water solution. The active pool of hydrogen ions is in equilibrium with the exchangeable hydrogen ions that are held on the soil’s cation exchange complex. This pool most readily affects plant growth. Active acidity may be directly determined using a pH meter, such as an electron probe.
- The second pool, exchangeable acidity, refers to the amount of acid cations, aluminum and hydrogen, occupied on the CEC. When the CEC of a soil is high but has a low base saturation, the soil becomes more resistant to pH changes. As a result, it will require larger additions of lime to neutralize the acidity. The soil is then buffered against pH change. (See base saturation discussion.)
-
Residual acidity comprises of all bound aluminum and hydrogen in soil minerals. Out of all pools, residual acidity is least available.
Buffering capacity
The quantity of aluminum and hydrogen in each of the 3 pools of acidity is not permanently fixed. Instead, the relative amounts of aluminum and hydrogen can change, as aluminum and hydrogen moves from pool to pool. Thus, the soil is said to have a buffering capacity. Buffering capacity is the ability of the soil to resist change. In the case of acidity, it is the ability of the soil to resist change in pH. Thus, aluminum and hydrogen of one pool will replenish the aluminum and hydrogen of another pool as these acid cations are removed.
For example, as aluminum and hydrogen are removed from soil solution, the acid cations of the CEC replenish the soil solution. Likewise, minerals containing aluminum and hydrogen dissolve and release these cations as they are removed from the exchangeable pool.
OUTLINE OF BUFFERING REACTIONS:
- Exchangeable acidity will buffer changes in active acidity
- Residual acidity will buffer changes in exchangeable and active acidity
Each soil has a unique buffering capacity. As a rule of thumb, finely-textured clay soils tend to have greater buffering capacities than coarse-textured soils.
Rule of Thumb
- Finely-textured clay soils tend to have greater buffering capacities than coarse-textured soils
Recall that 90% of Hawaii’s soils fall into this category. As a result, most Hawaii soils largely buffer soil acidity. This has great implications on nutrient management since buffering capacity determines the amount of resources, such as lime, that must be added to correct soil acidity. Soils that have high buffering capacities require larger amounts of liming resources to raise the pH to a target value than soils with low buffering capacities.
Problems associated with acidity in Maui County
The primary problems related to soil acidity in Hawaii are aluminum and manganese toxicities. Both toxicities, if not prevented, may cause severe damage to the crop and crop yield.
ALUMINUM TOXICITY
Aluminum toxicity can occur in soils that have large amounts of aluminum containing minerals. In such soils, aluminum can dissolve into the soil solution as the soil pH drops below 5.4. In contrast, aluminum solubility decreases dramatically as the soil pH increases above 5.4. As a result, proper management of soil pH can prevent problems associated with aluminum toxicity.
- Excessive amounts of aluminum can inhibit root development and limit crop growth.
- Aluminum saturation is an expression which describes the relative abundance of aluminum in the soil.
- Like base saturation, aluminum saturation is the percentage of the CEC occupies by aluminum. Like all cations, aluminum held by the cation exchange complex is in equilibrium with aluminum in the soil solution.
- Although the tolerance to aluminum varies among plant species, most plants do not tolerate greater than 15% aluminum saturation.
- However, certain crops grown in Hawaii, such as sugarcane, pineapple, corn and ti, can tolerant relatively high levels of aluminum saturation.
Conditions that cause aluminum toxicity
Aluminum toxicity occurs readily under acidic conditions, especially when pH values are equal to or less than 5.4. In the acidic soils of the tropics, aluminum toxicity may become a serious problem and limit crop yield. Management of soil pH is the key factor in avoiding aluminum toxicities. Aluminum toxicity may be ameliorated by liming your fields.
MANGANESE TOXICITY
Manganese toxicity can become a problem in soils with manganese-containing minerals. When these minerals dissolve, manganese ions are released into the soil solution. Although manganese is an essential plant nutrient, excessive quantities of manganese may be detrimental to plant growth.
Manganese toxicity symptoms include yellowing of leaves (chlorosis) of older leaves, which darken into small, brown spots. Although crop tolerance of manganese toxicity varies, most crops are sensitive to high levels of manganese. For example, manganese toxicity will result in the “sudden crash” syndrome of watermelon, in which plants suddenly wilt and die. Cases of “sudden crash” have been reported on O’ahu.
Conditions that cause manganese toxicity
Manganese toxicity can develop in soils that contain manganese-minerals. Moist, organic soils under acidic conditions are especially susceptible. Like aluminum toxicity, management of pH is very important. When the soil pH drops below 5.2, manganese minerals become highly soluble and perhaps toxic. Farmers may reduce manganese toxicity by liming and aerating fields. Aeration may be accomplished by irrigating less or draining water from fields.
Table 2. The effect of soil pH on aluminum and manganese in selected Oahu sugarcane fields.
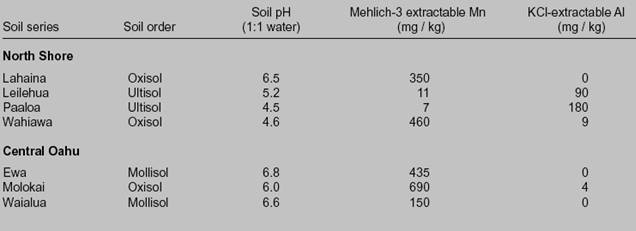
Source: Hue, N.V., J.A. Silva, G. Uehara, R.T. Hamasaki, R. Uchida, and P. Bunn. 1998. Managing manganese toxicity in
former sugarcane soils on Oahu. University of Hawaii at Manoa, College of Tropical Agriculture and Human
Resources, publication SCM-1. p. 7
For an excellent discussion of manganese toxicity, click on the following link. This publication includes a discussion of the effects of manganese toxicity on yield and provides management advice, as well as images of manganese toxicity symptoms:
http://www.ctahr.hawaii.edu/oc/freepubs/pdf/SCM-1.pdf
Management of soil acidity
Land managers can manage soil acidity by raising the pH to a desired value through several methods:
-
Flooding: In lowlands systems, flooding may be an effective technique in raising the pH of the soil. However, this effect is only good for the time for which the soil is flooded. Flooded or paddy mineral soils are ‘self-liming’. When they are flooded and become anaerobic (lack of oxygen in the soil atmosphere) for a period of time, the pH rises toward neutrality even when the soil pH was originally acidic. If the soil is subsequently drained and becomes more aerobic (more oxygen in the soil atmosphere), the pH will return to an acidic state
- However, care must be taken if the soil contains manganese-oxide minerals, since flooding conditions may lead to manganese toxicity.
- Crop consideration is also required. Flooding conditions reduces the oxygen within the soil, which is needed for plant life. As a result, crops that do not tolerate high amounts of water and low oxygen levels are not be suited for flooded conditions. Taro and rice are examples of crops that grow well in flooded lowlands.
-
Additions of organic matter: Additions of organic matter is a viable option to manage problems associated with soil acidity.
- Organic matter increases the cation exchange capacity of the soil. As the base saturation increases, the relative amount of “acid cations” decreases.
- In addition, organic matter forms strong bonds, known as “chelates,” with aluminum. Chelation reduces the solubility of aluminum and soil acidity. Again, if your soil is prone to manganese toxicity, it is not suggested that you add organic matter.
-
Additions of wood ash: Like organic mater, wood ash increases base saturation and forms chelates with aluminum.
-
Conventional Liming: Various liming materials may be added to the soil that neutralize, or counteract, soil acidity. Liming materials are bases that react with hydrogen ions in the soil solution to form water?
- Examples of common liming materials are limestone (calcium carbonate), dolomite (calcium/magnesium carbonate), hydrated lime (calcium hydroxide), and quicklime (calcium oxide). Calcium and magnesium silicates are also used as liming agents.
Liming
If you make the decision to apply lime to your soil, how much lime should you apply? Like other soil properties, the lime requirement will various depending upon the soil.
There are four guidelines that help us determined the lime requirement: the desired change in pH, buffering capacity of the specific soil, type of liming material, and the fineness or texture of the liming material.
-
Should you lime your soil? The optimal pH range for most plant is between 6.0-6.5. To avoid aluminum and manganese toxicity problems, a soil should be limed if the pH is less than 5.4.
-
What is you soil type? Recall the rule of thumb governing buffering capacity. Since finer textured soils have greater buffering capacity than coarser textured soils, more lime must be added to the finer textured soil to achieve the same effect and reach your target pH.
-
What type of liming material should you use? Due to differences in chemical composition and purity, liming materials have varying neutralizing strengths. See Table 5 below. As a result, you must know the neutralizing strength of your liming material before you can determine how much to add to the soil to reach a target pH.
-
What is the texture of your liming material? In general, the finer the liming material, the greater the neutralizing activity. However, application of extremely fine lime may be difficult, especially under windy conditions.
Calcium carbonate equivalent of liming materials
The calcium carbonate equivalent (CCE) is an important measurement in determining how much lime should be applied to your soil.
- By definition, CCE is the capacity of the liming material to neutralize acidity.
- CCE is expressed as a percentage of calcium carbonate, which serves as the standard (100%).
Table 3. Various liming materials and their relative neutralizing strengths
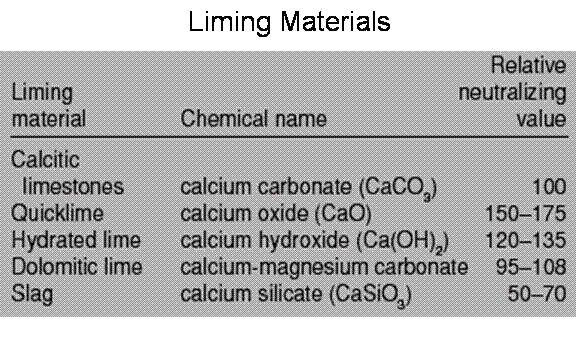
Source: Uchida, R.S., and N.V. Hue. Soil acidity and liming. p.101-111. In: Silva, J.A. and Uchida, R.S. (eds.) Plant Nutrient Management in Hawaii’s Soils: Approaches for Tropical and Subtropical Agriculture. College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa, Honolulu.
Neutralizing Exchangeable Aluminum
A direct benefit of liming your soil is to reduce aluminum saturation. Most plants suffer when aluminum saturation is greater than 15%. You can first determine the aluminum saturation through soil testing, and then determine how much lime is required to neutralize the exchangeable Al. This is particularly important in the tropics, where aluminum saturation can become high.
Liming curves
Easy to read and interpret, liming curves are very useful tools in determining the calcium carbonate equivalent that should be added to a particular soil to reach a target pH. Liming curves can be experimentally determined and calibrated for specific soils. CTAHR has published liming curves for selected soils of Hawaii and are available on the web.
To view liming curves for selected soils of Hawaii, click on the following two links below:
http://www.ctahr.hawaii.edu/oc/freepubs/pdf/AS-1.pdf
http://www.ctahr.hawaii.edu/oc/freepubs/pdf/pnm10.pdf
Application and Overliming
After uniform application to the soil surface, lime should be thoroughly tilled into the soil for optimal neutralization of soil acidity. Since the liming material can only react at the depth to which it is incorporated, subsurface tillage may be necessary to ameliorate subsoil acidity. Liming soils that contain with perennial crops can also be accomplished with surface application with little or no tillage. Irrigation and rainfall slowly leaches the lime, which is relatively insoluble, from the surface into the soil profile where it can react to neutralize soil acidity. Using the proper fertilizer formulation is an important way to manage soil acidification, particularly in perennial crop systems where liming is less easily incorporated than in annual or short-term systems.
Despite the many benefits of lime, over-liming your soil has an adverse effect. It is important to carefully considered how much to lime to add. If the pH of the soil is adjusted too high, it can induce nutrient deficiencies (such as phosphorus and micronutrient deficiencies), as well as permit molybdenum toxicity.
Gypsum
Gypsum is a soil conditioner that may be used to correct aluminum problems in the subsurface soil layers. However, it is important to make the distinction between lime and gypsum. Although both are sources of calcium, lime raises the pH of the soil, while gypsum does not.
Toolbox
To facilitate your liming and fertilization calculation, we provide easy-to-use calculators. These calculators enable immediate determination of the quantity of fertilizers or lime needed based upon your specific land dimensions and target nutrient levels. Additionally, since these calculators were created in an excel format, you can conveniently save your calculations in record archives.
The University of Hawai‘i is an equal opportunity/affirmative action institution.
copyright ©2007-2022 University of Hawai‘i - College of Tropical Agriculture and Human Resources

Home Soil Basics Soils of Maui Nutrient Management References
 Home > Nutrient Management > Essential Nutrients
Home > Nutrient Management > Essential Nutrients
Essential Nutrients
There are 15 essential elements that plants must have in order to grow properly.
18 Essential Nutrients
- Nutrient elements obtained from atmosphere through photosynthesis
- Nutrient elements obtained from the soil
- Nitrogen
- Phosphorus
- Potassium
- Sulfur
- Magnesium
- Calcium
- Iron
- Boron
- Manganese
- Zinc
- Molybdenum
- Copper
Out of the 15 essential elements that come from the soil, we deal with only the 12 that are generally managed by the growers. These 12 elements are ‘mineral nutrients’ and are obtained from the soil. We further divide mineral nutrients into 3 groups: primary, intermediate, and micronutrients. Our presentation will exclude cobalt, chlorine, and nickel from our discussion on the management of essential mineral nutrients, though are included by many as essential nutrients.
- The primary nutrients are nitrogen, phosphorus and potassium. You may be most familiar with these three nutrients because they are required in larger quantities than other nutrients. These three elements form the basis of the N-P-K label on commercial fertilizer bags. As a result, the management of these nutrients is very important. However, the primary nutrients are no more important than the other essential elements since all essential elements are required for plant growth. Remember that the ‘Law of the Minimum’ tells us that if deficient, any essential nutrient can become the controlling force in crop yield.
- The intermediate nutrients are sulfur, magnesium, and calcium. Together, primary and intermediate nutrients are referred to as macronutrients. Macronutrients are expressed as a certain percentage (%) of the total plant uptake. Although sulfur, magnesium, and calcium are called intermediate, these elements are not necessarily needed by plants in smaller quantities. In fact, phosphorus is required in the same amount as the intermediate nutrients, despite being a primary nutrient. Phosphorus is referred to as a primary nutrient because of the high frequency of soils that are deficient of this nutrient, rather than the amount of phosphorus that plants actually use for growth.
- The remaining essential elements are the micronutrients and are required in very small quantities. In comparison with macronutrients, the uptake of micronutrients is expressed in parts per million (ppm, where 10,000 ppm = 1.0%), rather than on a percentage basis. Again, this does not infer that micronutrients are of lesser importance. If any micronutrient is deficient, the growth of the entire plant will not reach maximum yield (Law of the Minimum).
Since the soil provides most essential nutrients, it is crucial that we understand the soil processes that determine the availability of each essential nutrient for plant uptake.
Table 4. Forms of Essential Elements Taken up by Plants
Element Abbreviation Form absorbed
Nitrogen N NH4+ (ammonium) and NO3- (nitrate)
Phosphorus P H2PO4- and HPO4-2 (orthophosphate)
Potassium K K+
Sulfur S SO4-2(sulfate)
Calcium Ca Ca+2
Magnesium Mg Mg+2
Iron Fe Fe+2 (ferrous) and Fe+3 (ferric)
Zinc Zn Zn+2
Manganese Mn Mn+2
Molybdenum Mo MoO4-2 (molybdate)
Copper Cu Cu+2
Boron B H3BO3 (boric acid) and H2BO3- (borate)
In this website, we will discuss major factors that affect the availability of the essential nutrients.
- In the tropics, the management of nitrogen and phosphorus can be problematic. Thus, it is appropriate that we discuss management issues of each nutrient separately.
- In a second section, we will collectively discuss the availability of potassium, calcium, and magnesium in Hawaii soils.
- Lastly, we will address issues of micronutrient management in the tropics.
- We will omit a discussion on sulfur, since it is seldom deficient in Hawaii soils.
<< Previous Next >>
The University of Hawai‘i is an equal opportunity/affirmative action institution.
copyright ©2007-2022 University of Hawai‘i - College of Tropical Agriculture and Human Resources
"