Do what you can to favor it. I had one similar to that on my pear around 10 years ago. Marked its limb and watched, but I wasn’t willing then to majorly cut back my tree (it was on a low internal branch that was shaded by the main canopy). It didn’t grow anymore and eventually died like any other shaded out branch. Prune to favor it, and hope the mutation is in the spur, and not just in the individual flower stem.
https://www.nature.com/articles/s41438-018-0062-x
" bud sport is a lateral shoot, inflorescence or single flower/fruit with a visibly different phenotype from the rest of the plant. The new phenotype is often caused by a stable somatic mutation in a single cell that is passed on to its clonal descendants and eventually populates part or all of a meristem. In many cases, a bud sport can be vegetatively propagated, thereby preserving the novel phenotype without sexual reproduction. Bud sports provide new characteristics while retaining the desirable qualities of the parent plant, which is why many bud sports have been developed into popular cultivars. We present an overview of the history of bud sports, the causes and methods of detecting somaclonal variation, and the types of mutant phenotypes that have arisen spontaneously. We focus on examples where the molecular or cytological changes causing the phenotype have been identified. Analysis of these sports has provided valuable insight into developmental processes, gene function and regulation, and in some cases has revealed new information about layer-specific roles of some genes. Examination of the molecular changes causing a phenotype and in some cases reversion back to the original state has contributed to our understanding of the mechanisms that drive genomic evolution.
Introduction
The use of traditional breeding to improve the quality of perennial fruit, nut, or ornamental plants is hindered by several factors. Many perennial species have a long juvenile period and generation time, some are self-incompatible, and most are highly heterozygous, which means valuable qualities may be lost through sexual reproduction. For these reasons, many woody perennials are vegetatively propagated by cutting, grafting, and budding, which can preserve desirable genotypes over long periods of time. Indeed, there are examples of wine grapes that have been clonally propagated for centuries1,2.
Occasionally, a lateral shoot, inflorescence or single flower/fruit is discovered with a visibly different phenotype from the rest of the plant. These are called bud sports and are often caused by a stable somatic mutation in a single cell that is passed on to its clonal descendants and eventually populates part or all of a meristem, enabling vegetative propagation of the new mutant. Although these are relatively rare events, sport mutations often provide valuable new characteristics while retaining the desirable qualities of the parent plant. Therefore, somatic mutation represents a mechanism to generate new genetic variability which is especially important for species with low levels of variation. Many economically important perennial cultivars are bud sports3,4. By 1936 there were at least 1664 known fruit tree bud sports, representing 32% of the plant patents issued by the U.S. Patent Office at that time5. More recently, Okie6 reported that more than 170 commercialized cultivars of peach and nectarine are derived from bud sport mutations.
The identification of bud sports relies on astute observation by the grower, breeder, or gardener. While these have likely been observed since humans have been cultivating plants, the earliest report of a bud sport was published by the botanist Gaspard Bauhin in 1598 and describes the unusual leaf phenotype of a Chelidonium majus (celandine) plant found in a herb garden7. In 1644, gardener Pietro Nati noticed a shoot bearing unusual fruit (the “Bizarria” orange) growing from the graft junction of two types of citrus8. Charles Darwin was fascinated by “sporting plants” and published numerous reports of spontaneous mutants9,10,11,12. In his famous book, The Variation of Plants and Animals Under Domestication 11, Darwin noted that “Many cases have been recorded of a whole plant, or a single branch, or bud suddenly producing flowers different from the proper type in colour, form, size, doubleness, or other character. Half the flower, or a smaller segment sometimes changes colour”. In some cases, the molecular or cytological change(s) causing the new phenotype have been identified, which has contributed to our knowledge of the mechanisms that lead to the formation of sports and provided novel information about gene function and regulation. This review will highlight some of these examples.
Meristems make the plant
Most of the above-ground parts of the plant are produced by clusters of rapidly dividing cells in the apical and axillary meristems. Angiosperm meristems are organized into one or more outer layers, the tunica, and an inner layer or corpus which reflect stereotypical patterns of cell division gleaned from histological analysis and cell lineage studies13. Cells in the tunica tend to divide anticlinally (new cell walls formed perpendicular to the surface) such that they form one or more clonally distinct layers (Fig. 1). The number of tunica layers ranges from one to five with most species having two, the L1 and L2. The L1 gives rise to the epidermis, the L2 generates sub-epidermal layers and the germline. The corpus or L3 divides in all planes and gives rise to cells that become the core of lateral organs and the cortex of the stem.
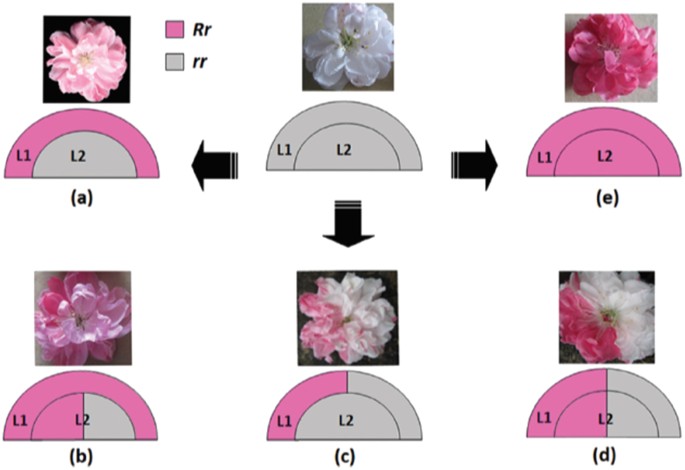
Fig. 1: A proposed model for the variegated phenotype in flower colouration in peach cv. HBH.
L1 and L2 indicate different layers of floral meristems, and R and r represent functional and non-functional alleles of the RIANT gene, respectively. White flower carrying two non-functional alleles of the RIANT gene (rr). a Pink flower derived from a periclinal chimera. b Pink flower with red somatic sectors derived from a mericlinal chimera. c White flower with pink somatic sectors derived from a mericlinal chimera. d White flower with red somatic sectors derived from a sectorial chimera. e Red flowering carrying one functional and one non-functional allele of the RIANT gene. Source: Cheng et al.61
Superimposed over the tunica/corpus organization are distinct zones that are defined by histological properties and cell division rates. At the very apex of each layer, there are a very small number of cells that are larger and stain less densely than surrounding cells13. These initial cells divide slowly and act as a reservoir of stem cells that replenish the supply of rapidly dividing cells on the flanks and central zone of the meristem that eventually become incorporated into lateral organs and the stem13.
The analysis of chimeric plants comprised of cells with distinct genotypes and phenotypes has provided valuable insight into meristem organization14. For example, a somatic mutation occurring in a cell located close to the apex often results in a mericlinal sector, which is visible in a portion of an organ (Fig. 1b, c, d). Occasionally, a mutant cell will populate an entire layer, creating a periclinal or layer chimera that can remain stable for long periods of time (Fig. 1a–c). Adventitious shoots originating at graft junctions can produce graft chimeras, which are generally periclinal chimeras comprised of two distinct genotypes or even different species15,16,17,18. Two excellent reviews provide in-depth information about the use of both spontaneous and induced chimeras as a research tool19 and as a source of valuable new horticultural cultivars20. Mutant L2 periclinal chimeras enter the germline, and thereafter can be sexually propagated, leading to the mutation becoming fixed in subsequent generations. Periclinal chimeras can also become homogenized when mutant cells divide into adjacent layers and eventually displace wild-type cells.
It has been assumed that meristematic cells accumulate more mutations because they have high rates of mitosis. However, it has recently been shown that shoot apical meristem cells have a relatively low mutation rate even in large perennial plants21,22, which may explain the low rates of somatic variation found in long-lived trees23.
Causes and identification of somaclonal variation
Mutations can be caused by changes to the DNA sequence or by epigenetic variations that modify DNA or histones and affect gene expression, but do not alter the sequence itself. DNA sequence errors may occur during replication, recombination, DNA damage repair during mitosis, and by transposable elements (TE). Additionally, epigenetic variations such as DNA methylation, histone modification, chromatin remodelling, and RNA silencing can cause stable changes in gene expression, generating sports.
The most frequent mutations caused by DNA polymerase errors are point mutations, tandem repeats, small insertions and deletions (indels), and base mismatches. Polymerase slippage is known to produce variability in simple sequence repeat (SSR) regions. For example, Aranzana and co-workers24 estimated a mutation rate of 1.1% of SSR alleles between peach sports. More recently, high-throughput next-generation sequencing (NGS) technologies have been used to estimate variability between bud mutants, providing more comprehensive information. Whole-genome sequences of “Fuji” apple and four bud mutant cultivars were compared to identify small polymorphisms, which revealed an average rate of eight single-nucleotide polymorphisms (SNPs) and 1.2 indels per Mb over the genome25. In grape, Carrier et al.26 compared whole-genome sequences of Pinot noir and three of its clones and found 1.6 SNPs and 5.1 indels per Mb.
Failures in the DNA replication process and damage caused by chemicals or radiation may produce DNA double-strand breaks (DSBs). Eukaryotic organisms have developed efficient mechanisms to repair DSBs through non-homologous end joining (NHEJ) and homologous recombination (HR) pathways. NHEJ is likely to occur more frequently than HR27 and does not require a homologous sequence to ligate the two DSB; it can result in small insertions or deletions of DNA at the break location and presumably contributes to microsatellite instability28. In contrast, the HR pathway requires an intact DNA molecule as template, such as the sister chromatid in a cell in S or G2 phases or the homologous chromosome. While recombination with the sister chromatid will not produce a mutation, recombination with the homologous chromosome may result in loss of heterozygosity or genome rearrangements29,30. Migliaro et al.31 characterized grape sports that were likely generated after independent DSBs and subsequent repair produced deletions ranging in size from a single bp to larger than Mbs along chromosome 2. More complex structural variations have been identified on three chromosomes of the grape sport Tempranillo blanco32, resembling a chromothripsis-like mechanism (clustered chromosomal rearrangements), which could have been generated by illegitimate re-joining of chromosome breaks in a unique event. Another example of structural variations that can alter gene expression and phenotype are copy number variants, which involve duplications or deletions of large segments of DNA33,34.
TEs contribute to genome plasticity and cause the majority of somatic variation in plants25,26,35,36,37. TEs can disrupt coding sequences, alter the expression of nearby genes and produce chromosome breakage leading to genome rearrangement and/or genome instability38. Retrotransposons are one class of TEs that are particularly disruptive because they replicate via reverse transcription of messenger RNAs and the duplicates integrate into other chromosomal locations. Related long terminal repeat (LTR) transposons are also sources of somatic variability as they can excise due to either homologous or illegitimate recombination between the terminal repeats, resulting in genomic loss or rearrangement. The activation and silencing of TEs is regulated by epigenetic mechanisms, therefore changes in such mechanisms may produce TE-driven somatic variability. Transcriptomic and epigenetic analyses have demonstrated variation in the transcription of genes associated with epigenetic mechanisms during bud dormancy release in fruit trees39,40, which may lead to the generation of sports. Stresses such as wounding, pruning, viral infection, and tissue culture are all known to induce the movement and/or activity of TEs41,42, as well as DNA damage43. McClintock44 first proposed that stress-induced activation of TEs could be a plant survival strategy to rapidly increase genotypic and phenotypic diversity in response to unfavourable conditions.
In most cases, bud sport mutations are genetically identical to their parent except for the new mutation; comparison of the parent and sport genotypes provides an opportunity to identify the molecular lesion responsible for the new phenotype. Several molecular and sequencing methods accompanied by bioinformatics pipelines have been developed to detect novel somatic changes. NGS technologies, especially those producing long, single-molecule reads using nanopore sequencing, have proven reliable in the detection of TEs that are transpositionally active in plants45, while new strategies are proposed to identify TEs active during plant development46. For a more precise estimation of somatic variability rates, such methods should consider the layer specificity of some mutations as in Marroni et al47.
Sports can arise from plants that are heterozygous for one or more loss-of-function mutation(s) that acquire independent new mutation(s) to the functional allele. Dominant gain-of-function mutations or a loss-of-function mutation to a haploinsufficient gene (one that requires two functional alleles to appear wild type) can produce a different phenotype with a single mutation event.
Lastly, not all bud sports are caused by genetic mutation. Hybridization between species and spontaneous changes in ploidy can introduce somatic variability leading to novel phenotypes48. Many sports originate as chimeras, comprised of cells from two distinct genotypes14,20. Most of the bud sports identified affect the fruit, probably because they are easy to observe, and many ornamental sports have altered floral or leaf phenotypes. In the sections that follow, we will group sports by their altered phenotype.
Floral and inflorescence morphology and/or colour
Humans have appreciated the beauty, colour and aromatic scent of flowers since at least 12,000 years ago49. Many ornamental plant cultivars originated as bud sports that change the appearance of flowers or inflorescences. Wild roses have a single whorl of five petals, whereas most cultivated roses (Rosa hybrida) have many petals. Dubois50 and co-workers demonstrated that the rose orthologue of AGAMOUS (RhAG) is expressed in whorls 3 and 4 of wild roses, consistent with its role as a C-function gene that has a key role in specifying stamen and carpel identity51. In double-flower roses, RhAG expression is restricted to a much smaller domain in the centre of the floral meristem and whorls of stamens are converted to petals. Analysis of sports that revert back to five petals also show expanded RhAG expression into whorl 350. The molecular lesion causing the misexpression of RhAG is unknown; identification of the mutation responsible may reveal information about the regulation of RhAG expression. The authors suggest that the boundary between A-function and C-function gene expression is very labile and might explain how double flower roses have arisen and been selected by humans more than twice.
Somatic grape mutants with altered flower and inflorescence development produce some of the most conspicuous phenotypes and illustrate the developmental plasticity of the tendril52. A somatic variant of Carignan has a reiterated reproductive meristem (RRM) phenotype, leading to large, highly branched fruit clusters and delayed anthesis. The tendrils of RRM mutants are also more indeterminate than wild type, displaying multiple branches or even conversion to a leafy shoot. The RRM phenotype is caused by insertion of a TE into the promoter of VvTFL1A, a close homologue of Arabidopsis TERMINAL FLOWER1 (TFL)35. Genetic and genomic analyses demonstrate that the insertion of the transposon is associated with upregulation of VvTFL1A. This is consistent with previous studies showing that TFL genes control the length of developmental phases and maintain indeterminate inflorescence growth53,54.
Mutants of Gamay, Morrastel, and Pinot initiate extra whorls of sepals and petals and are collectively known as multiple perianth whorls52. Stamen and carpel development is abnormal in variants of Bouchalès and Mourvèdre, the latter being completely sterile. Although the molecular lesion(s) causing these phenotypes is unknown, the MADS box ABC genes would be prime candidates55.
More than half of commercial varieties of azalea (Rhododendron simsii hybrids) are colour sports56. Solid colour and variegated sports are likely L1 mutants because the new phenotype is not transmitted to progeny57. The variegated sports are likely to be transposon-mediated changes to pigment genes, although no direct evidence exists. The “picotee” phenotype is characterized by petals with a coloured centre and white margins. In broad-margined “picotee” mutants, the margin cells were found to be tetraploid and the coloured cells diploid, suggesting that positionally determined polyploidisation underlies this pattern. In general, coloured azalea sports were hypermethylated relative to their parent57. In carnation (Dianthus caryophyllus), the L1 layers showed very different patterns of methylation to L2 and L3 layers, and these patterns differed widely between sports and their parents58.
Ornamental flowering peach trees can produce white, pink, and red flowers on the same tree59,60,61. In some cases, this variability has been attributed to an unstable TE in the W locus60 or differential expression of transcription factors and genes in the anthocyanin biosynthesis pathway between red and white flowers59, but genetic lesions responsible have not been identified. In the “Hongbaihuatao” (HBH) cultivar, Cheng61 and co-workers identified a small indel in RIANT, a gene encoding an anthocyanin transporter required for pigment accumulation. White flowers are homozygous for a 2-bp insertion which introduces a frameshift mutation and a premature stop codon. Red and pink flowers are heterozygous at the RIANT locus, with one non-functional allele and a second allele with either a 1-bp insertion or a 2-bp deletion that restores gene function. Periclinal, mericlinal, and sectorial chimeras with or without RIANT function produce white, pink, and red flowers (Fig. 1). These mutations are not in a microsatellite region, but are in a CG-rich region, which could increase the rate of small indels62.
Pollination, seedlessness, and fruit ripening
Self-incompatibility (SI) is a genetic mechanism that prevents inbreeding in some flowering plants. In most plants in which this SI mechanism operates, SI is controlled by a single, multi-allelic S-locus, which enables the pistil to reject pollen with the same S-allele63. Self-compatible sports have been identified in Japanese pear (Pyrus serotina)64 and almond (Prunus delcis)65. The pear sport is caused by genomic deletion of at least 4 kb that removes an S-RNase gene responsible for the SI reaction in the style. This mutation is only in the L1, providing evidence that the transmitting tissue in pear is L1-derived. In the self-compatible almond sport “Jeffries”, at least two mutations occurred, the deletion of one S haplotype and duplication of another, resulting in self-compatibility.
In most species, fruit and seed development are linked; however, there are examples where fruit development occurs in the absence of seed development. Other forms of SI occur after pollen germination and affect fertilization or embryo development, often leading to the development of a seedless fruit. Numerous seedless citrus sports have been identified, some of which have become popular cultivars such as satsuma mandarin (Citrus reticulata) and seedless navel orange. The Zigui shatian pummelo (Citrus grandis Osbeck) sport produces self-pollinated fruit, but the seeds are sterile because of defective post-zygotic development66. The seedless mandarin sports Ougan67, Wuzishatangju68, and Huami Wugegonggan69 are caused by pollen abortion, blocked fertilization, and pollen sterility and embryo abortion, respectively.
Bud sports that ripen earlier or later than their parents have been identified in numerous species. While many aspects of fruit ripening are directly controlled by the hormone ethylene70,71, different genotypes vary in their sensitivity to ethylene72. Fruit types with ripening traits predominantly regulated by ethylene are often termed “climacteric”, while those that are predominantly regulated by other factors are labelled “non-climacteric”. Genomic sequencing of bud sports of the Japanese plum “Santa Rosa” revealed copy number variation in genes associated with ethylene perception and signal transduction73. The non-climacteric and supressed-climacteric mutants had significantly fewer copies of 1-aminocyclopropane-1-carboxylic acid (ACC) oxidase (the enzyme that catalyses the final step in ethylene synthesis) and the ethylene receptor gene ETHYLENE INSENSITIVE 1 (ETR1) relative to their climacteric parent. A number of frameshift mutations were also identified in genes involved with sugar transport and ethylene biosynthesis. Similarly, early-ripening “Beni Shogun” apples show increased expression of ethylene synthesis and signal transduction genes74. Late-ripening Tardivo mandarin sports are less sensitive to ethylene and have decreased expression of ETR1 and ETR2 75,76. Transcriptomic and proteomic analyses of several late-maturing bud sports of sweet orange have revealed differential expression of genes involved with abscisic acid (ABA), ethylene, and jasmonic acid (JA) synthesis and signal transduction, as well as sugar metabolism and carotenoid biosynthesis77,78,79.
Altered fruit colour
Humans have been propagating grapevine (Vitis vinifera L.) for fresh fruit and wine making for over 10,000 years and from the second half of the 20th century clonal selection for wine grape breeding has been intensively used, so it is not surprising that many somatic variants have been identified that affect berry quality traits (mainly colour) and that these sports have been developed into cultivars. Grape berry colour is caused by the accumulation of anthocyanins in the berry skin (L1) and flesh (L2)80. Two tandemly repeated Myb regulatory genes, VvMybA1 and VvMybA2, regulate red berry colour81,82,83. Mutations and instability affecting this locus are the molecular basis for the majority of grape colour sports.
White grapes are thought to have originated from a black-fruit ancestor via two independent mutations to the closely linked VvMybA1 and VvMybA2 genes. A Gret1 retrotransposon insertion into the promoter of VvMybA1 and a small indel causing a frameshift mutation in VvMybA2 inactivate both genes82,83. It is unclear exactly when the heterozygous red parent self-pollinated and gave rise to homozygous white progeny, but there is evidence that ancient Egyptians were making both red and white wine by 1332 BC84. Many of the white grape cultivars tested are homozygous for these same two mutations suggesting that most have a common origin82,83, 85,86,87. Loss of the Gret1 transposon in some sports restores VvMybA1 function and gives rise to coloured revertants82,86,88. Other loss-of-pigment sports have been shown to arise from insertion of other types of TEs near VvMybA26, short genomic insertions into the promoter or introns of VvMybA89, or large-scale genomic replacements and rearrangements near VvMybA32,90.
Dark skinned cultivars occasionally produce a bronze or pale coloured sport that eventually gives rise to white berries. Walker and co-workers demonstrated the molecular basis of two such examples91. Cabernet Sauvignon produces dark red berries, but is heterozygous for the mutant VvMybA genes described above. A new mutation causing a large deletion of the functional VvMybA genes occurred in the L2 of Malian, abolishing anthocyanin production in this layer and giving the berries a bronze colour. Malian is unstable and occasionally produces white grapes or white sectors following an invasion of mutant L2 cells into the L1 (Fig
"
I have yet to review but greatly appreciate this! I’m sure questions are to come.
It looks like a gardenia bloom!
Oh wow, I love Kieffers too! Damn, this one’s gonna be a banger! 
Clark, if it sets a fruit, please let it grow. There is a high probability the seed produced will inherit the mutation.
Mutations occur at a relatively predictable (rare but it happens) rate. Most somatic mutations affect just the meristem tissue which means the small bud of growing tissue at the tip of a developing branch. These mutations may cause a change in flower or fruit color, flower or fruit shape, fruit flavor, or other detectable traits. Mutations that affect flower shape may not be passed on through the growing tip the flower is on. In other words, only the flower may be affected.
A flower mutation may be genetic (can be inherited), epigenetic (might be inherited as a partially expressed trait in the next generation) or epistatic (affects only the current flower and may never recur).
It is very likely that this flower is from a fused ovule which would explain the petal doubling.
What a beautiful flower! Bud sports and mutations are inherently interesting and where many interesting varieties come from. Good job on so much of the heavy science. I wish I could understand half of it. I think that seckel pears are a hybrid of part of a relatively unknown species.
The tornado going through Kansas reminds me of a movie.
The name of it, “Rosebud”, reminds me of another movie. Maybe you could make it the last line of a movie.
John S
PDX OR
Just like tbudding i’m thinning back leaves simulating a hungry herbivore. Branch is coming out of the bud just like it understood what we wanted. See why I say prune carefully on a pear this was an anticipated result. If your pruning for fruit and not branches you might have made a mistake already. The bud and branch are in God’s hands ofcourse. It did not set fruit and it wasn’t expected. Let’s hope that branch is the same as that bud. Clipped that missing leaf because I know what pears do in response to that.
rosacea…those blooms say it all
Maybe someday people can look at a tree covered with blooms like this in the spring! Seems that beauty would make life better.

It is very unfortunate this sport died. There was nothing i could do about it. At least we have the images of this truly beautiful pear. My guess is whatever caused the branch to die caused the mutation.
AW man! And such a beauty… at least you got that picture!
Thank you for sharing those beautiful pictures, Clark. A real treat to be able to enjoy such things, even if only for a short time.
For people who are interested, this seems like a good place to mention that Fedco offers a pear that is reputed to have double blossoms.
Cabot Vermont European Pear
Pyrus communis Fall. An old dessert pear, circa 1850, discovered in Cabot, Vermont, a few miles west of the New Hampshire border and about as far north as Bangor, Maine.
A superior dessert pear with medium-large oblong pear-shaped fruit. Yellowish skin has a slight reddish blush. Yellowish sweet flesh is coarse grained, extremely juicy, with no grit cells. Not only is it a delicious dessert fruit, it is also remarkable for its very rare “double” flowers. Highly ornamental! Introduced to us many years ago by Armando Bona of Passumpsic, Vermont. Not to be confused with the old Massachusetts pear Cabot.
I got some scionwood from Fedco a couple of years ago and grafted a couple of trees of Cabot Vermont, so far successfully. Probably a few years away from blossoms yet but I will try to post some pictures if they do come out double.
Interesting. Fedco displayed really not beautiful pears picture but didn’t have any picture of the beautiful blossom on its website
Seems like a missed opportunity, doesn’t it?
Yes i would have liked to have cut that bud off and tbudded it and maybe i should have but my chance would have only increased to 50%. Thought i was playing it safer.
Sorry, Clark, I’m afraid that my comment may have come across as armchair pear-pruning and it wasn’t intended as such. I was actually responding to Annie’s remark about Fedco’s decision to show a picture of the (somewhat scruffy) Cabot pears but not the blossoms.
In any case, no need to second-guess yourself. Thank you for sharing the pictures, and as always, your knowledge and enthusiasm.
Every once in a while a plant will repeat a similar mutation. That particular tree already has a genetic code for double blooms. It just needs that little mutation to unlock it. It’s possible an existing bud nearby also has the mutation, but will only show if it pushes growth.
I’ve been watching for it. If nothing else it was beautiful for that time we saw it.
And I appreciate that you let us see it too.