This website does a good job of explaining callusing and grafting to the fruit grower who wants the detailed version of how grafting working
(https://www.frontiersin.org/articles/10.3389/fpls.2020.590847/full)
Grafting is a common practice for vegetative propagation and trait improvement in horticultural plants. A general prerequisite for successful grafting and long term survival of grafted plants is taxonomic proximity between the root stock and scion. For the success of a grafting operation, rootstock and scion should essentially be closely related. Interaction between the rootstock and scion involves complex physiological-biochemical and molecular mechanisms. Successful graft union formation involves a series of steps viz., lining up of vascular cambium, generation of a wound healing response, callus bridge formation, followed by vascular cambium formation and subsequent formation of the secondary xylem and phloem. For grafted trees compatibility between the rootstock/scion is the most essential factor for their better performance and longevity. Graft incompatibility occurs on account of a number of factors including of unfavorable physiological responses across the graft union, transmission of virus or phytoplasma and anatomical deformities of vascular tissue at the graft junction. In order to avoid the incompatibility problems, it is important to predict the same at an early stage. Phytohormones, especially auxins regulate key events in graft union formation between the rootstock and scion, while others function to facilitate the signaling pathways. Transport of macro as well as micro molecules across long distances results in phenotypic variation shown by grafted plants, therefore grafting can be used to determine the pattern and rate of recurrence of this transport. A better understanding of rootstock scion interactions, endogenous growth substances, soil or climatic factors needs to be studied, which would facilitate efficient selection and use of rootstocks in the future. Protein, hormones, mRNA and small RNA transport across the junction is currently emerging as an important mechanism which controls the stock/scion communication and simultaneously may play a crucial role in understanding the physiology of grafting more precisely. This review provides an understanding of the physiological, biochemical and molecular basis underlying grafting with special reference to horticultural plants.
Introduction
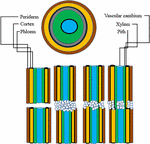
Grafting has been performed in agriculture since the beginning of civilization. Historical records have revealed that ancient Chinese and Greeks have been practicing it since 1560 B.C. (Melnyk and Meyerowitz, 2015). Since fruit and nut trees are difficult to propagate by cuttings, grafting is used for their propagation. Moreover, the superiority and quality of the grafted crops further led to widespread adoption of this technique. It is a well-established practice which makes it possible to physically join two or more genetic entities in a single tree to influence the productivity characters of a tree favorably and facilitates asexual propagation in horticultural crops like apple, pear, plum and cherry (Figures 1A–D; Kumari et al., 2015). A “de novo” formed meristematic area must develop between scion and rootstock for a successful graft union. The scion becomes the new shoot system and the rootstock (under stock, stock) forms the root system of the grafted plant. Scions are selected based on yield related traits and are generally grafted over specific rootstocks having the ability to survive the biotic and abiotic components of the environment. Rootstock mostly influences scion vigor and its water relations. Grafting is usually practiced in perennial horticultural trees with the main aim to reduce vegetative growth and shorten the juvenile period. Additional benefits of grafting include dwarf tree structures to increase the planting density per unit area and hence productivity simultaneously with minimal investments in orchard cultural practices like pruning, pest and foliar disease control. For an efficient root system to develop the rootstock and scion compatibility plays a crucial role (Goldschmidt, 2014; Warschefsky et al., 2016). However, rootstock and scion compatibility vary to an extent that even the closely related species might not be compatible therefore, it becomes necessary to evaluate the compatibility before grafting a particular scion into a rootstock (Lee et al., 2010; Guan et al., 2012). Success of a grafting operation depends on the strength of the union formed. Stronger unions result in successful grafting operation. On the contrary, weaker unions lead to graft failure and in adverse cases the trees may fall apart. Graft union formation depends on a number of factors viz., molecular pathways and physiological/biochemical responses. Lot of effort has been put into studying the physiological mechanism of union formation, causes and consequences of graft incompatibility and also as to how molecules are being transferred across the graft unions to reveal the mechanisms responsible for inducing the phenotypic changes by grafting. In this review we not only get an idea about the fundamental mechanism of graft union formation, graft incompatibility: its types, mechanism and causes, but it also makes some of the critical molecular and physiological mechanisms associated with grafting much easier for us to understand.
FIGURE 1
Figure 1. (A) Grafted Apple (Rootstock- M9, grafting method-Wedge grafting, Age-7 years); (B) Grafted Pear (Rootstock- Quince- C, grafting method- Wedge grafting, Age-8 years); (C) Plum (Rootstock- Seedling, grafting method- Tongue grafting, Age-5 years); (D) Cherry (Rootstock- Gisela 5, Grafting method- Tongue grafting, Age-6 years).
Grafting Techniques in Fruit Trees
Grafting has become immensely important in view of improving cultivation especially of fruit and vegetable crops. Besides this, grafting tends to improve adaptability and resistance of plants to different environments and stresses (Kumar et al., 2017). The latter can be achieved by using a suitable rootstock. However, not every grafting operation is successful. Apart from a number of factors including stock scion combination used, season, temperature, etc., the technique of grafting followed is the most important one to determine the degree of grafting success (Soleimani et al., 2010). Grafting techniques include side, tongue, cleft, bark, and splice grafting methods (Figure 2). Among these cleft grafting also known as wedge grafting is the most commonly used method. Additionally, budding is also a form of grafting in which the scion size is reduced to a small piece of stem with one or more axillary bud attached to it. In general, success of a grafting operation depends on combining anatomical structures of the stock and scion, so much so that if there is some dislocation of vascular elements, weak or distorted unions may get formed eventually leading to graft failure (Wang, 2011). Thus, the choice of an appropriate grafting method is critical to ensure proper contact between stock and scion and to avoid the formation of incompatible or weak graft unions. Depending upon the existing environmental conditions the success of any grafting method may vary from one crop to another. Maximum success percentage i.e., 100% was obtained in mango by following cleft grafting technique in the month of June or March. On the other hand, Allan et al. (2010) reported that in papaya side grafting brings about 80% success rate while Nguyen and Yen (2018) recommended cleft grafting using 1-month old rootstocks as the best method for maximum grafting success in papaya. Maximum graft success in plum i.e., 9.67 out of 10 grafts was achieved by following cleft grafting in April suggesting it to be the commercial method for grafting (Mozumder et al., 2017). In walnut wedge grafting was found comparatively superior to tongue grafting in terms of sprouting percentage, graft union success, and plant growth (Srivastava, 2012). The influence of grafting technique on plant growth has been studied in peach cultivar Shan-e-Punjab grafted on wild peach rootstock. Different grafting methods including tongue grafting, chip budding and T budding were followed. Tongue grafted plants showed maximum sprouting percentage, graft success, plant height, girth and number of branches. The results indicate that tongue grafting is the best method of propagation for peach variety Shan-e-Punjab (Sharma et al., 2018). Bud take and bud sprouting were found to be earliest when T budding (with wood) was performed in cherry. Also, maximum shoot length and the highest number of leaves and lateral branches were obtained with T budding (with wood) compared to the other two methods i.e., T without wood budding and chip budding (Yazdani et al., 2016). Graft take success in apple cultivars grafted on different rootstocks was evaluated and it was found that the cultivar Gala mast grafted on crab apple rootstock by means of bench grafting showed maximum graft take success as well as prominent growth (Fazal et al., 2014). Whip and cleft grafting methods have been reported to be the most promising ones for the asexual propagation of Jabuticabeira Acu grafted on rootstocks belonging to other species of the same family (Cassol et al., 2017). Depending upon the research purposes other grafting methods like in vitro grafting have been introduced. Besides being time consuming, the planting material used in the conventional system of plant propagation is not healthy. To overcome these problems application of in vitro techniques is an effective alternative. One such application is shoot tip grafting or micrografting. Micrografting involves in vitro placing of shoot tip as an explant on a decapitated rootstock grown from a seed (Hussain et al., 2014). Micrografting protocols have been developed for many fruit crops including grapes (Aazami and Bagher, 2010), walnut (Wang et al., 2010), almond (Yıldırım et al., 2013), etc. Rafail and Mosleh (2010) reported that in in vitro grafting of apple and pear, homografting was relatively more successful compared to heterografting and an increase in micrograft success was noticed from 30 to 90% in pear (cv. Aly-sur on Calleryana pear) and 40 to 90% in apple (cv. Anna on MM106) with increasing benzylaminopurine (BAP) concentration from 0 to 2.0 mg/L. Patharnakh shoot tips were propagated in vitro on Kainth rootstock. Graft success and vigor was found to be maximum by following wedge grafting method and using 5–10 mm scions and M2 (MS liquid media +20 g/l sucrose) media (Rehman and Gill, 2014). Hetero-grafting allows the alteration of important plant processes including water uptake, nutrient absorption, hormonal signaling and enzyme activity. Cookson and Ollat (2013) reported that it is heterografting and not homografting which affects the gene expression pattern in shoot apical regions. Stress response genes were upregulated at the graft interface of heterografts compared to homograft’s indicating that the cells at the graft interface have the ability to recognize and function differently as soon as they come in contact with a self or non-self-grafting partner (Cookson et al., 2014). Comparative analysis of differentially expressed genes in homo and heterografted tomato seedlings was carried out and it was found that in heterografts healing process was slightly slow compared to homograft’s and several genes involved in oxidative stress were significantly up-regulated in the scion of heterografted tomato (Wang et al., 2019). Gene expression studies in homo and heterografts of grapevine revealed upregulation of genes involved in the synthesis of cell wall, wound responses, hormone signaling and other metabolic pathways in homograft’s, while in heterografts stress responsive genes were up-regulated at the graft interface (Clemente Moreno et al., 2014). Studies on efficiency of grafting techniques and time of grafting have been conducted and standardized for different areas (Ghosh and Bera, 2015). This information may help in determining ideal grafting time for quick multiplication of fruit crops to enhance quality fruit production. In view of the same, application of in vitro grafting techniques for propagation of fruit crops can be considered a relatively sustainable alternative to conventional methods of propagation. Micrografting technique has a great scope in plant improvement and their large-scale propagation. Production of virus free plants is one important advantage of this technique due to which it finds application in fruit crop propagation. In addition to this, prediction of graft incompatibility has been possible through micrografting. Grafting operation can be conducted at any time of the year through micrografting. Due to an adequate number of advantages this technology has great potential to be practically used by researchers and nursery growers.
FIGURE 2
Figure 2. Different types of grafting like side, tongue, cleft, bark, and splice.
Graft Union Formation
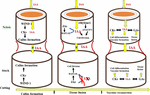
For grafting to be successful, a number of complex biochemical and structural processes are involved. The latter result in establishing a connection between the root-stock and scion. Adhesion of parenchyma is the first step for union formation followed by formation of vascular elements and their differentiation into xylem and phloem. Formation of vascular connection between the stock and scion during wound healing is of utmost importance as the wound given to the stock and scion during grafting causes disruption of the vascular system in plants (Asahina and Satoh, 2015), hence connecting up of the vascular system is required to facilitate water uptake as well as to ensure nutrient transport to the graft junction. In addition to this, vascular reconstruction enables macromolecules to be transported across the graft union (Harada, 2010). This specifies that vascular differentiation is imperative for grafting success during the process of wound healing. Five histological stages are reported to come about during graft union formation in rootstock scion combinations: (1) formation and orientation of necrotic layers, (2) callus cell proliferation, (3) formation of callus bridge at the graft interface, (4) vascular cambium formation and (5) vascular tissue reconstruction between the stock and scion (Figure 3). Except for the outer cortex necrotic layers tend to disappear by the cellular activities in the callus. In most of the cases the portion of necrotic layers in the outer cortex gets transformed into bark (Yildirim et al., 2010). The process of graft union formation is temporally separated. Herbaceous plants take relatively shorter time for the successful formation of a graft union compared to trees. Time required for graft union formation in the Arabidopsis micrograft system is 7–12 days (Turnbull, 2010). On the contrary, trees take several months to form a union at the graft interface (Olmstead et al., 2006). The process of vascular reconnection during the formation of a graft union has been studied in Arabidopsis thaliana. Attachment of tissues on either side of the cut surfaces, establishment of a connection between the phloem cells of rootstock and scion, resumption of root growth, and connection between xylem vessels were found to be temporally separated. It was found that at first the two parts get attached to each other, this is followed by connecting up of phloem about 3 days after germination (DAG), growth of the roots gets resumed at this stage around 5 DAG, and at 7 DAG xylem vessels get re-joined. By analyzing the pattern of cell division and tissue regeneration at the graft junction it was observed that cells at first showed an asymmetrical pattern of cell division and differentiation but as soon as a contact establishes between the stock and scion, they tend to lose this asymmetry and the vascular connection gets re-established (Melnyk et al., 2015). Melnyk et al. (2018) in their subsequent study explained in detail the differential expression and upregulation of many genes during the union formation leading to vascular regeneration. They described that genes are expressed asymmetrically at the graft junction in Arabidopsis hypocotyl grafts. This differential expression of genes was observed on account of abundant carbon concentration in the scion and less carbon in the rootstock, till the phloem was reconnected. At the graft union, genes associated with the formation of vascular tissues were upregulated, thereby activating a recognition mechanism between the stock and the scion. Different stages of union formation at the graft interface in tomato seedlings revealed that a number of structures appeared to interconnect the stock and scion 8 DAG, vascular bridges appeared at 11 DAG and connection between the root-stock and scion got completely established 14 DAG (Fan et al., 2015). On the other hand, histological stages of development of graft union in spur type apple varieties grafted on different apple rootstocks revealed that ample amounts of callus boomed in all the stock-scion combinations. Formation of cambium and reconnection of vascular cells was apparently successful in 90-day old grafts. Callus bridge filled the stock and scion gap on 120th day and it continued for a few more days, following which xylem and phloem strands bridged the union (Polat et al., 2010). Also, study of changes at the graft union in cashew by Mahunu et al. (2012) revealed that 30 DAG the necrotic layer disappeared, coinciding with the enlargement of callus, while adhesion of stock and scion occurred at 60 DAG. At 98 DAG cambial connections and healed unions were visible. However, in case of unsuccessful graft combinations at 98 days after grafting, a gap between the cells of stock and scion was noticed indicating that union formation is the key factor for graft success and further growth of the grafted plant. Since grafting puts a considerable amount of stress on plants, it is associated with the stimulation of a number of wounding responses such as production of ROS (reactive oxygen species), upregulation of certain genes providing stress resistance, synthesis of enzymes and other chemical substances. These compounds eventually trigger the formation of wound induced callus. Furthermore, the growing callus is sustained by production and stimulation of specific metabolites. Study of transcriptional changes at the graft interface in grapevine at 3 and 28 DAG revealed differential expression of certain genes involved in the synthesis of cell wall and formation of vascular elements. Expression of these genes was particularly upregulated at the graft interface compared to the rootstock which resulted in the identification of genes specific to the graft interface (Cookson et al., 2013). To identify the compounds involved in callus formation, Prodhomme et al. (2019) conducted metabolite profiling in grapevine. The study revealed increased production of amino acids (basic and branched chain) as well as accumulation of stilbene compounds at the graft interface. Additionally, the union formation was associated with increased activity of two enzymes viz., PAL and NI at the graft interface compared to the surrounding tissue. All these metabolic modifications together support callus growth and serve as a source for the identification of potential markers for selection in breeding programs. Despite these findings, we still lack the understanding of how the two components i.e., stock and scion actually establish a physical connection, integration of the vascular tissues, role of plasmodesmata in union formation, and material exchange at the graft interface. Thus, advanced research is needed to address these basic questions. This can be done by using fluorescent markers and correlative light-electron microscopy techniques (Gautier et al., 2019).
FIGURE 3
Figure 3. Represents stages of graft union formation: Stage 1, Parenchymatous tissue divides to form callus cells; Stage 2, Xylem vessel formation; Stage 3, Formation of vascular cambium across the graft union linking the two partners; Stage 4, Secondary xylem and phloem dedifferentiate across the graft union establishing sufficient vascular continuity for plant growth [Hartmann and Kester, (2002)].
Genetic Limits of Grafting
A prerequisite for graft compatibility is taxonomic proximity. Autografts are taxonomically quite close and thus are expected to be always compatible. When grafting is performed within the same species it forms compatible combinations, if the grafting partners are from different species but the genus to which they belong is same, grafts are more or less compatible, intra familial grafts are rarely compatible, while inter familial grafts are essentially unsuccessful due to incompatibility (Mudge et al., 2009). Therefore, the taxonomic proximity between the grafting partners is essential for the successful re-establishment of both the rootstock and scion fused together. It is a very well-known fact that presence of vascular cambium is a prerequisite for grafting. Vascular cambium due to its meristematic activity divides to form xylem and phloem during the secondary growth resulting in increased plant diameter (Spicer and Groover, 2010). Monocots cannot be grafted, moreover grafting of a monocot plant onto a dicotyledonous plant is also not possible. This is because vascular bundles in monocots are scattered and they lack cambium, which is a basic requirement for graft union formation. The parallel venation in the leaves of monocots indicates that the veins do not interconnect to each other like they do in dicots. Thus, it is the lack of vein connection in stem and leaves of monocot plants which makes grafting in monocots an impossible task (Figure 4). Plants that are closely related have a good chance of successful union formation compared to the remotely related ones. Such plants have less or no chance of successful graft union formation. In order to achieve maximum success, grafting should be performed between or within the clones (Kumar, 2011). However, successful interfamilial graft combinations between Nicotiana benthamiana (Nb) and Arabidopsis thaliana (At) have been reported where the growth of Nb scion was slow but distinct at the same time (Notaguchi et al., 2015). During the course of time, plants have developed a specialized haustorium that pierces into the host plant to derive nutrients by means of tissue adhesion and this property of cell to cell adhesion can be used to develop interfamilial grafts (Westwood et al., 2010). Natural tendency for cell to cell adhesion with plants belonging to different families including vegetables, fruits trees as well as monocots is found in Nicotiana. Here, the reconstruction of vascular structures follows the normal pattern as in case of intrafamilial grafting. A transcriptomic study revealed the upregulation of β-1,4-glucanases followed by graft adhesion in inter as well as intra familial grafts. The use of Nicotiana stem, an interscion produced tomato fruits on rootstocks belonging to different families. Therefore, the mechanism of cell to cell adhesion can be used to modify plant grafting techniques and to develop graft combinations which are otherwise difficult to obtain (Notaguchi et al., 2020).
FIGURE 4
Figure 4. It shows correlation between taxonomic proximity and graft compatibility. Grafting between closely related plants is comparatively more successful than distantly related ones. Maximum grafting success is achieved by performing grafting within or between the clones. Grafting success goes on decreasing as the plants become less related taxonomically.
Graft Incompatibility: Types and Determining Techniques
Graft incompatibility is generally referred to as inability of the stock and scion to bind together to form a successful graft union. Lack of compatibility between the rootstock and scion is the major limiting factor in propagation of fruit trees, particularly stone fruits (Zarrouk et al., 2006). Graft incompatibility is therefore a critical issue for breeding rootstocks of fruit trees and longevity of an orchard (Hossein et al., 2008). It leads to the formation of unhealthy trees, breakage at the graft union and premature death of grafted plants (Zarrouk et al., 2006). The arrival of these symptoms could take a number of years (Guclu and Koyuncu, 2012). Thus, to ensure a successful graft union the selection of a mutually compatible scion/rootstock combination is important (Goldschmidt, 2014).
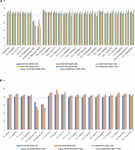
Graft incompatibility is broadly categorized as: translocated and localized (Zarrouk et al., 2006). In case of “translocated” incompatibility, symptoms are observed at early stages of plant development. Scion and root growth tend to terminate at a very early stage, reduced carbohydrate translocation at the union, shriveling of leaves, leaf chlorosis leading to leaf reddening and early leaf drop are commonly observed symptoms. Translocated incompatibility can be evaluated using the soil plant analysis development (SPAD) chlorophyll meter which measures the chlorophyll content and nitrogen status of plants. Low SPAD index values indicate restricted carbohydrate assimilation and nitrogen uptake due to translocated incompatibility (Zarrouk et al., 2006). Significantly lower SPAD index values were observed for rootstocks “Mirabolano 29C” and “Marianna 2624” in the three scion cultivars, ultimately resulting in death of the trees owing to incompatibility between the graft partners. The rootstocks showing translocated graft incompatibility symptoms like reddening of leaves, excessive leaf curling, leaf drop, etc., with scion cultivars died 5 months after planting. SPAD index values did not decrease in other scion/rootstock combinations after 9 months of planting, indicative of their compatibility (Figures 5A,B; Neves et al., 2017). Thus, SPAD chlorophyll meter serves as an effective and non-destructive tool for the prediction of incompatible graft combinations.
FIGURE 5
Figure 5. (A) SPAD values for Jade and Maciel (scion cultivars) grafted onto a range of clonal rootstocks. (B) SPAD values BRS-Kampai scions grafted on a range of clonal rootstocks (Neves et al., 2017).
Localized incompatibility, on the other hand, leads to malformation at the graft union due to physiological and morphological changes taking place which eventually results in impaired union formation and in severe cases the tree might fall apart at the junction after some years of grafting (Errea, 1998). The alterations associated with localized incompatibility include disruption of vascular cambium, lower rate of tissue differentiation, lignification may not take place efficiently and disruption of vascular continuity. These changes might cause the graft union to rupture (Zarrouk et al., 2006, 2010; Pina et al., 2012, 2017). The most known example of localized incompatibility is between pear and quince, when pear cultivars are used as scion and quince as rootstock, prunasin, a cyanogenic glycoside generally present in quince but absent in pear is translocated into the phloem cells in pear scion, where the pear enzymes disrupt the prunasin in the region of graft union, producing hydrocyanic acid as one of the products of decomposition. Hydrocyanic acid obstructs the actively dividing cambial cells at the graft union and also disrupts phloem tissues at and above the graft union. Restriction in water flow and mineral/metabolite translocation across the union consequently kills quince phloem as well (Gur et al., 1968). Early and correct forecast of graft incompatibility is of utmost significance because the unwanted incompatible combinations could be avoided while the desirable compatible ones could be selected (Petkou et al., 2004; Gökbayrak et al., 2007). Standardized methods for evaluation of compatibility between the rootstock and scion would be of great use to the breeders while using a particular rootstock and scion for grafting (Pina et al., 2017). In several apricot combinations grafted on prunus rootstocks, graft incompatibility resulted in breakdown of the trees at the union years after planting, therefore an early selection process could help in detecting a comparatively compatible combination. Analysis of the phenol content at the graft union can be used as a technique for the estimation of graft incompatibility (Dogra et al., 2018). Callus formation is of utmost importance for stock and scion to be compatible and grafting to be successful. In grapevine it was found that it is not the graft take rates but the status of callus formation at 21 DAG which is an indicative of compatibility between the stock and scion. Moreover, analysis of leaf chlorophyll content can also serve as an efficient means to estimate the compatibility (Tedesco et al., 2020). The difference in the quality and quantity of phenol in the stock and scion can point toward metabolic dysfunctions at the graft union (Errea, 1998) and can serve as a biochemical marker to predict graft incompatibility (Musacchi et al., 2000). Histological studies of callus formation and cell alignment have made it possible to predict the compatibility of any combination way before the symptoms appear (Errea and Borruey, 2004). Additionally, the findings of Guclu and Koyuncu (2012) revealed that by using peroxidase activity as a technique it is possible to predict graft incompatibility before the grafting operation is conducted particularly in those combinations that might show delayed incompatibility symptoms (Figure 6). Furthermore, electrophoresis method and X-ray tomography can serve as important tools for assessment of graft quality and success (Dogra et al., 2018). The complex mechanisms involved in the incompatibility reaction between the stock and scion have been studied in many ways, however, still many processes remain unclear therefore, advanced research is needed to completely understand the physiology of graft incompatibility especially in perennial plants.
FIGURE 6
Figure 6. Overgrowth above the union caused by blockage of photosynthates, translocating from the scion into the stock, when sweet cherry is grafted onto sour cherry [(A) Age 10–12 years; (B) Age 7–8 years].
Role of Phenolic Compounds in Graft Incompatibility
Incompatibility is allied with complex biochemical and physiological interactions between the grafting partners (Pereira et al., 2014). Pina and Errea (2008), suggested that graft compatibility/incompatibility response could be related to the protein UDP-glucose pyrophosphorylase. Phenolic substances play a crucial part in plants and are one of the most important compounds that determine the rootstock-scion interactions. Errea (1998) believed that quality and quantity of phenols in rootstock-scion parts indicated why the rate of metabolic activities is low at the graft union. Mng’omba et al. (2008) presented that the combinations which are apparently less compatible possessed high concentrations of phenolic compounds than the compatible ones. r-coumaric acid was present in a huge amount in relatively less compatible combinations. Therefore, phenols particularly r-coumaric acids and flavonoids resulted in weak union formation at the graft junction. This is the peculiar sign of graft incompatibility. Pear stock-scion combinations were found associated with profuse amounts of arbutin in phloem above and below the graft union, after that procyanidin B1 and chlorogenic acid. Compatible combinations had greater arbutin levels above the graft junction, whereas in the incompatible combinations of “Williams” on quince MA high arbutin concentration was recorded at the lower side of the graft union. In all the cultivars under study, arbutin content was found to be highest below the graft union specifying that it’s not just catechin and procyanidin B1, but also arbutin and a number of flavonols may possibly serve as a cause for graft incompatibility (Hudina et al., 2014). Several studies have shown that phenolic compounds in incompatible combinations move from vacuole to cytoplasm and cause inhibition of lignification which is required during early stages of establishment of scion–stock connections. The cell wall of xylem vessels are dynamic in nature composed of phenolic compounds (for example, lignins), minerals, polysaccharides and proteins (Liu, 2012; Herrero et al., 2014). These phenolic compounds can serve as important markers for determining compatibility between different graft combinations (Prabpree et al., 2018). The role of different cyanogenic glycosides (CGs) in the incompatibility reaction of Prunus has been evaluated in different graft combinations belonging to prunus species viz., Prunus persica and Prunus mume and ungrafted genotypes. The incompatible graft combinations were found to have greater prunasin levels and activity of the enzyme phenylalanine ammonia lyase (PAL) was also higher in rootstock. Additionally, the scion and stock were found to have a moderately higher concentration of total phenolic compounds with high antioxidant activity. Differences in concentration of CGs, primarily prunasin, was found to be responsible for the incompatibility between Prunus persica and Prunus mume (Pereira et al., 2018). Therefore, grafting between such plants with great differences in CG concentrations may end up in generating incompatibility reactions between the partners (Pereira et al., 2015). Plant hormones, especially auxins determine the compatibility of a rootstock-scion combination by interacting with phenolic compounds. Incompatibility has been associated with increased levels of phenolic compounds above the graft union which adversely affect the auxin transport (Errea, 1998). Low auxin concentration in incompatible combinations in turn affect the differentiation of vascular tissues and lignification (Aloni, 2010; Koepke and Dhingra, 2013). All these changes will lead to the formation of weak unions which may cause huge economic losses to the growers. More information about the compounds responsible for inducing graft incompatibility is needed. This knowledge is necessary for the development of molecular markers for rootstock breeding (Gainza et al., 2015).
Hormonal Control of Graft Union
The development of successful grafts involves some fundamental steps in the following pattern: phloem tissue reunion, root growth, and xylem tissue reconnection (Melnyk et al., 2015). The key event involved in the formation of a graft is the joining of vascular tissues between the rootstock and scion. Grafting first and foremost causes the discharge of pectins from cells at the graft union which makes the rootstock and scion adhere together. Dedifferentiated cells at the union form callus at the graft interface, these cells then interdigitate and form a connection via plasmodesmata. The vascular tissues differentiate together with the callus at the grafting site giving rise to phloem which is succeeded by reconnection of xylem tissue (Jeffree and Yeoman, 1983; Melnyk et al., 2015; Ribeiro et al., 2015). Plant hormones commonly known as phytohormones regulate all phases of growth and development in plants, from embryogenesis to reproductive development. They standardize the response of plants to a wide range of biotic and abiotic stresses. Additionally, phytohormones regulate the physiological processes taking place at the site of graft union. Phytohormones facilitate plants to combat the stress induced by grafting. Aloni (1995) found that relatively less concentration of indole-3-acetic acid (IAA) encouraged phloem differentiation, but higher levels brought about the differentiation of xylem. Similarly, in grafting trials, auxins are an essential group of elements resulting in the formation of compatible graft unions (Figure 7). Auxins are produced from the vascular strands of the stock and scion, which bring about the vascular tissue differentiation, hence working as a growth regulating substance (Aloni, 1987; Mattsson et al., 2003). Plant hormones are translocated from source to sink as signal molecules influencing growth of cells and differentiation of vascular tissues, especially at the graft crossing point (Aloni, 2010). Kümpers and Bishopp (2015) demonstrated that phytohormones regulate the complex physiological interaction between scions and rootstocks in A. thaliana. Along these lines, they may be well thought-out candidates in the scion–rootstock communication both above and below the grafted interface (Kondo et al., 2014). Nanda and Melnyk (2018) inspected the function of eight plant hormones for the period of grafting to determine their role in healing of cut surfaces and vascular tissue differentiation in plants at the graft interface (Melnyk, 2017). They concluded that every known plant hormone controls the vascular tissue differentiation during the period of graft union formation. Nonetheless, auxin is the principal regulator of vascular differentiation and other hormones augment its signaling pathway to make satisfactory adjustments in this process. After the cut is given, wound induced dedifferentiation 1 (WIND1) stimulates cytokines which triggers the formation of callus at the graft junction. Simultaneously, auxin gets transported basipetally and due to the lack of vascular connectivity, its flow across the union is hampered, resulting in its accumulation above the graft interface and low concentration at the bottom junction. Auxin accumulation, along with ethylene signaling, triggers the expression of Arabidopsis NAC domain containing protein 71 (ANAC071) above the graft union, and simultaneously inhibits the expression of RAP2.6L as well as Jasmonic acid biosynthesis. Beneath the graft, the depleted auxin levels trigger the biosynthesis of Jasmonic acid and expression of RAP2.6L. The expression of ANAC071 and RAP2.6L prompts cell division around the graft junction. Auxin, in collaboration with gibberellins and cytokinins, stimulates differentiation of cells, resulting in formation of vascular connection and re-joining between both junctions, thereby restoring auxin symmetry. Gibberellins, in collaboration with auxin, stimulate emergence of tissues by means of cell expansion (Figure 7; Nanda and Melnyk, 2018).
FIGURE 7
Figure 7. Hormonal signaling taking place at the graft interface during the grafting process. CKs, cytokinins; IAA, auxin; 534 JAs, Jasmonic acids; GAs, Gibberellins.fpls-11-590847.pdf (6.0 MB)